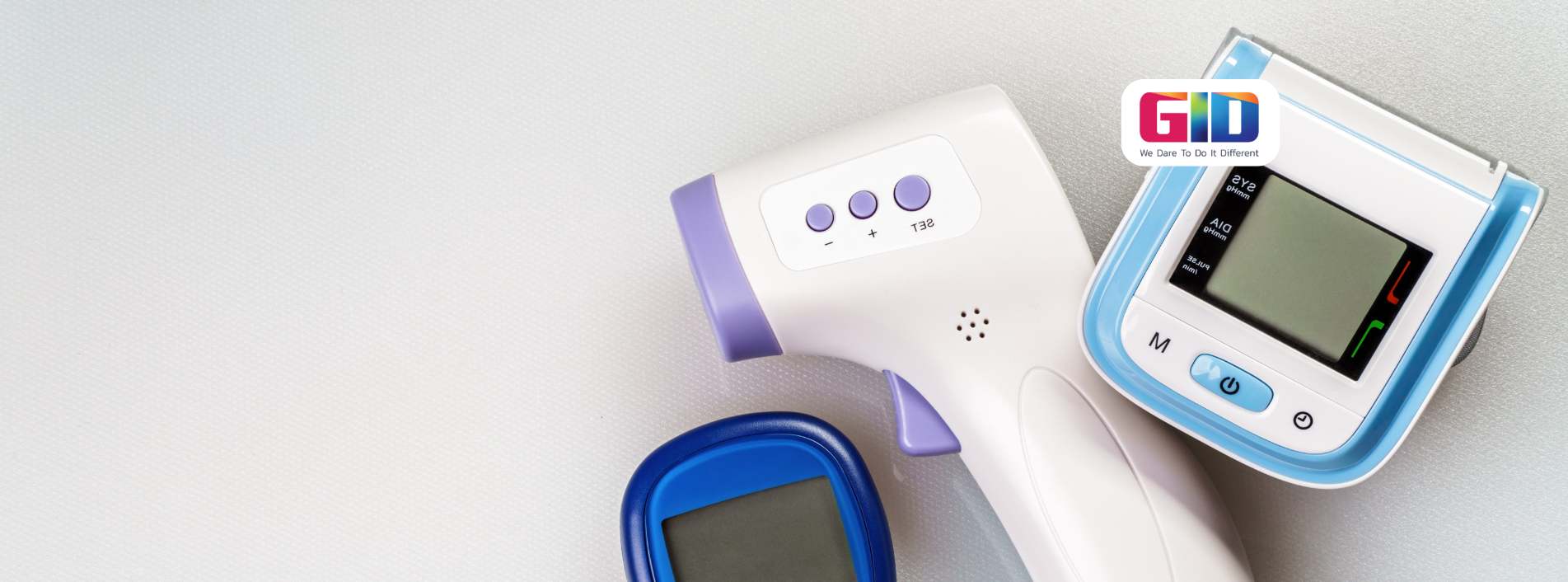
In the world of healthcare, medical devices are essential tools for improving patient outcomes. These devices range from simple surgical instruments to sophisticated diagnostic tools, all designed to enhance the quality of care. Behind every groundbreaking medical device lies a complex yet fascinating manufacturing process. Let’s explore this intricate world to understand how these life-saving devices are brought to life.
The Medical Device Manufacturing Journey
The journey of a medical device from concept to clinical use is a carefully orchestrated process that encompasses various stages, each contributing to the device’s safety, effectiveness, and reliability.
Designing the Device
The first step is to design the device, where engineers and designers work with healthcare professionals to identify unmet clinical needs and translate them into tangible concepts. This stage involves brainstorming, research, and sketching to transform ideas into detailed blueprints.
Building Prototypes
Once the design is finalized, prototypes are created. These serve as physical representations of the device, allowing for testing and refinement before committing to full-scale production. Prototypes are iteratively improved until the desired functionality and user experience are achieved.
Rigorous Testing
To ensure safety and efficacy, medical devices undergo a series of tests. They are evaluated for performance, durability, and biocompatibility in controlled laboratory environments and clinical trials. These tests ensure that the devices meet stringent regulatory standards and pose no harm to patients.
Full-Scale Production
With the design perfected and testing complete, full-scale production begins. This involves selecting the most appropriate manufacturing methods, establishing quality control measures, and ensuring a consistent supply of raw materials. Production facilities are designed to maintain a sterile environment and adhere to strict manufacturing protocols.
Quality Assurance and Regulatory Compliance
Quality assurance and regulatory compliance are paramount in medical device manufacturing. Strict regulations govern the design, manufacturing, and distribution of these devices to protect patient safety and ensure that only the highest quality devices reach the market.
Maintaining Quality
Quality assurance is an ongoing process that encompasses all aspects of medical device manufacturing, from design to production. It involves implementing quality control measures, conducting regular audits, and maintaining meticulous documentation. These measures ensure that every device meets the highest standards of quality and safety.
Adhering to Regulations
Medical device manufacturers must comply with a complex set of regulations established by governing bodies like the FDA. These regulations outline the requirements for design, testing, production, and labeling of medical devices. Compliance with these regulations is essential for obtaining market approval and ensuring that devices meet the highest standards of safety and efficacy.
Emerging Trends and Innovations
The field of medical device manufacturing is constantly evolving, driven by technological advancements and the pursuit of better patient outcomes. Emerging trends and innovations are shaping the future of this industry, with the potential to revolutionize healthcare delivery.
Additive Manufacturing (3D Printing)
Additive manufacturing, also known as 3D printing, is transforming the way medical devices are manufactured. This technology allows for the creation of complex geometries and customized devices, enabling personalized treatment approaches.
Artificial Intelligence (AI)
AI is rapidly gaining traction in medical device manufacturing, with applications in design optimization, quality control, and predictive maintenance. AI algorithms can analyze vast amounts of data to identify patterns, predict potential failures, and optimize manufacturing processes.
Robotics
Robotics is becoming increasingly integrated into medical device manufacturing, automating tasks that are repetitive, hazardous, or require high precision. Robotic systems can enhance production efficiency, reduce human error, and improve worker safety.
GID Company: Your Partner in Medical Device Development
While GID Company doesn’t directly manufacture medical devices, we can be an invaluable partner in bringing your medical device ideas to life. Our expertise in product design, prototyping, and regulatory compliance can help you navigate the complexities of medical device development and bring your innovative products to market faster and more efficiently.
Conclusion
Medical device manufacturing is a complex and multifaceted process that plays a crucial role in advancing healthcare and improving patient lives. As technology continues to evolve and the demand for innovative medical devices grows, the field of medical device manufacturing will continue to expand and play an even more significant role in shaping the future of healthcare. You can Call Jim at +1714-323-1052 or You can like our Facebook page and follow us on X as well.